CRISPR-Cas9 has taken the world by storm with the promise of making gene editing much easier and faster than ever before. But how does CRISPR actually work? How can biology research benefit from it? What will happen when we start using it to edit human DNA? And what’s the fight between its developers all about?
CRISPR-Cas9 is one of the biggest discoveries of the 21st century. Since it was developed in 2012, this gene-editing tool has revolutionized biology research, making it easier to study disease and faster to discover drugs. The technology is also significantly impacting the development of crops, foods, and industrial fermentation processes. Genome Editing In Animals
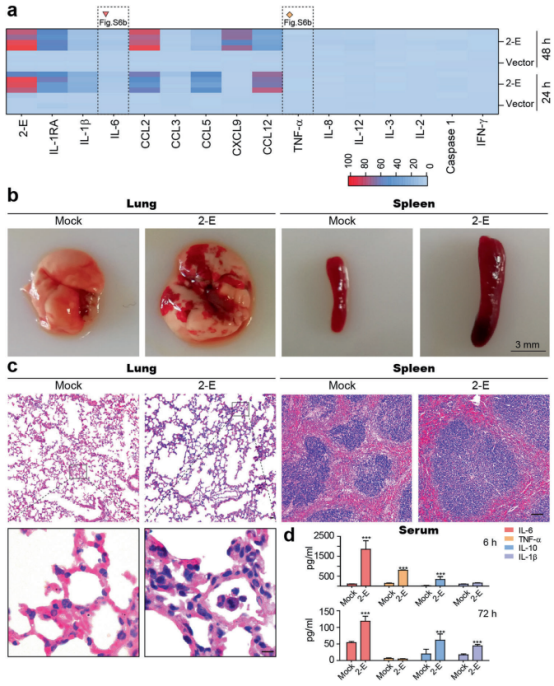
But the one application that has made it famous is the modification of the human genome, which brings the promise of using CRISPR to cure diseases such as HIV. The first clinical trials testing CRISPR-Cas9 in people are already underway in China, Europe, and the US. So while scientists start venturing into tweaking our own DNA, it is worth taking the time to fully understand what CRISPR is, and what the actual benefits and risks of using the technology are.
CRISPR is short for ‘clustered regularly interspaced short palindromic repeats.’ The term makes reference to a series of repetitive patterns in the DNA of bacteria and archaea that were extensively researched by Spanish scientist Francis Mojica in the ‘90s.
These patterns are the basis of a primitive immune system that bacteria use to ‘remember’ the DNA of viral invaders by incorporating the DNA sequence of the virus within the CRISPR patterns. The Cas9 protein is then able to recognize the DNA sequence stored within CRISPR patterns and cut any DNA molecules with a matching sequence.
But it wasn’t until 2012 that Jennifer Doudna and Emmanuelle Charpentier took the discovery a step further and proposed that CRISPR-Cas9 could be used to cut any desired DNA sequence by just providing it with the right template. Another two papers published just a few months later by Feng Zhang and George Church from the Broad Institute also reported some early uses of CRISPR as a gene-editing tool.
It is important to note that CRISPR is by far not the first system that allows us to edit DNA in all sorts of organisms. Other gene-editing technologies used extensively before are TALEN and zinc-finger nucleases (ZFNs). In fact, some experts point out that these tools, which have been in use for enough time to become quite refined, are more accurate than CRISPR-Cas9.
But CRISPR brings an important advantage over these other techniques: it is much easier and faster to use. Most previous technologies required creating a gene-editing protein from scratch for each specific DNA modification. With CRISPR, the same Cas9 molecule can be directed to any sequence just by providing it with a guide RNA molecule, which is much easier to synthesize. Companies like Synthego in the US have spotted a good business opportunity producing these guide molecules for researchers.
In theory, CRISPR gene editing could be used to make any modification to the DNA of virtually any living being. In biotech and pharma companies, CRISPR is becoming the go-to tool for drug discovery and development. In academic research labs, the gene-editing tool is being used to modify the genome of all sorts of organisms to study the function of any gene of interest, whether it is one that causes disease or one that makes a crop grow faster or survive harsh conditions.
In agriculture, CRISPR could be used to produce crops with better yields or that can resist drought, much faster than is possible with traditional breeding techniques. It can also be used to add new features, such as making tomatoes spicy, or to remove others — for example making gluten-free wheat or decaf coffee beans.
However, regulations can limit the use of these technologies. While the US has already seen the launch of CRISPR-modified crops, the European Union decided to set strict GMO regulations that scientists believe are hindering the potential of the technology.
But right now, most of the money seems to be in using CRISPR-Cas9 to engineer human DNA. With over 10,000 diseases caused by mutations in a single human gene, CRISPR offers hope to cure all of them by repairing any genetic error behind them.
There are two main approaches to using CRISPR as a therapy. The first is called ex vivo gene editing. It involves extracting human cells, engineering them in the lab, and reinjecting them into the patient. This method is similar to that used for most gene therapies already on the market. However, it can become quite expensive given each patient requires an individual manufacturing process for their therapy.
The second method is called in vivo gene editing and involves delivering CRISPR-Cas9 into the patient’s body to edit the DNA directly from within the cells. CRISPR could be delivered inside nanoparticles or encoded into DNA and be cleared out of the body once it has completed its mission. This method using CRISPR has only started human testing in 2020, and there are some concerns that there is a risk of CRISPR making off-target modifications.
Those are the big questions right now. Especially after CRISPR gene editing was controversially used to create the world’s first gene-edited babies in 2018. These ‘CRISPR babies’ carry a mutation intended to protect them against HIV infection. The experiment resulted in a strong pushback as scientists around the world questioned the ethics of altering human DNA without fully understanding the possible consequences. Indeed, there was a study suggesting that people carrying these mutations might be at risk of catching certain infections and dying younger.
Despite all the controversy around CRISPR therapy and the large amounts of money invested in it, we are still in the early stages of clinical trials. Scientists are wary of repeating the same mistakes as when gene therapy was first tested in humans back in the ‘90s, resulting in the death of 18-year old Jesse Gelsinger and causing years of delay in the development of gene therapy.
Now, even if CRISPR proves to be safe in humans, is it ethical to modify the human genome? The first applications of the technology, aimed at curing genetic diseases, seem quite straightforward. But where should the line be drawn? At what point does a therapy become a tool for eugenics?
Although the point in time when we are able to modify all sorts of human features at will is far ahead in the future, it is never too early to start thinking about how the technology should be regulated. Many scientists, including Jennifer Doudna, seem to agree that we should stay away from germline editing — that is, any modifications that children will inherit. At least for now.
Since the first publications showcased CRISPR-Cas9 as a gene-editing tool back in 2012, a number of companies have been set up by the developers of the technology.
Based in Switzerland and the US, there is CRISPR Therapeutics, co-founded by Emmanuelle Charpentier. Working in partnership with Vertex Pharmaceuticals, the company already has preliminary results from two ongoing phase I/II trials showing promise for the treatment of the blood disorders β-thalassemia and sickle cell disease. CRISPR Therapeutics is using an ex vivo approach where the bone marrow stem cells of the patient are genetically engineered outside the body.
In the US, the first CRISPR clinical trial started in early 2019, run by scientists at the University of Pennsylvania. On top of these academic efforts, US firms like Intellia Therapeutics, co-founded by Jennifer Doudna, are working on CRISPR-based treatments. Intellia’s first target is an in vivo treatment for a rare neurological disease called transthyretin amyloidosis. The company has already dosed its first patient with the therapy and the phase I trial is ongoing.
Founded by Doudna and Charpentier’s competitor Feng Zhang, there is also Editas Medicine, working in therapies for genetic blindness and cancer, among others. Doudna originally co-founded Editas along with Zhang but stopped all involvement just a few weeks after Zhang was granted his CRISPR patent and issues concerning intellectual property began to appear.
“The intellectual property in this space is pretty complex, to put it nicely,” said Rodger Novak, co-founder and previous CEO of CRISPR Therapeutics. “Everyone knows there are conflicting claims.”
The team of Doudna and Charpentier at UC Berkeley filed a first patent application for CRISPR in May 2012, a few months before their paper was published. Zhang and the Broad Institute filed theirs in December that year, but they paid the US patent office to fast-track the review process. This resulted in Zhang’s patents being issued before there was a decision on his competitors’.
UC Berkeley then initiated a process to invalidate the Broad’s patent on the basis that Doudna and Charpentier had developed the technology and applied for a CRISPR patent earlier. The US patent office ended up ruling in favor of the Broad Institute after both parties combined had already spent over $20M (€16M) in legal fees.
“It reminds me of reading about really unhappy rich people,” said George Church about the patent fight. “They have such a big blank check that they just make each other miserable.”
“Everything here is very exaggerated because this is one of those unique cases of a technology that people can really pick up easily, and it’s changing researchers’ lives. Things are happening fast, maybe a bit too fast,” commented Charpentier. “I am very confident that the future will clarify the situation. And I would like to believe the story is going to end up well.”
Indeed, the situation is quite favorable for Charpentier and Doudna across the Atlantic. In Europe, they have secured broad patents on CRISPR while the Broad Institute saw its patents revoked in early 2020. While the Broad can still enforce its intellectual property in Europe with narrower claims, its licensing and royalty fees may be lower.
“The situation is paralyzing small companies. They are afraid of being held liable for patent infringement so they’d rather not use the technology,” said Ulrich Storz, Senior Partner at Michalski Hütterman Patent Attorneys. “This situation is not very common in biotech. … We weren’t prepared, and that’s why there have been so many problems with this technology, with this patent challenge.”
In short, the patent situation around CRISPR remains messy. Even today, the battle is still ongoing and it could take years for clarity to emerge.
With its potential already demonstrated in research applications, the next big milestone for CRISPR will be to prove it is safe and effective as a treatment. But there are still many other applications underway.
For example, the US company eGenesis, co-founded by George Church, is using CRISPR to modify the pig genome so that their organs can be transplanted into humans without rejection.
Another is the use of CRISPR as a diagnostics tool; some researchers have already developed a method to detect Covid-19 using CRISPR.
Other companies, like CRISPR Therapeutics and US biotech Allogene Therapeutics, are using CRISPR to develop gene-edited T cell therapies from donor cells. However, Allogene recently suffered a setback after it found an unexpected genetic change in a patient participating in its phase I/II clinical trial. The company has had to pause all clinical development as a result.
Nevertheless, CRISPR might still surprise us as new variants are developed. Swiss scientists have developed a method to simultaneously edit up to 25 genes using CRISPR. And another version of the gene-editing tool called CRISPR-Cpf1 that makes it easier to replace one DNA sequence with another is already being used by big brands such as BASF.
“You can imagine that many labs — including our own — are busily looking at other variants and how they work,” Doudna said. “So stay tuned.”
The gene-editing tool is also becoming very popular among DIY scientists and biohackers. Some believe that the relatively simple methods that this technique requires might help democratize science and bring it closer to people outside the lab. However, it remains to be seen how these uses will be regulated.
In any case, the impact of CRISPR in biology is already tangible and it has already gone down in history as a big discovery. The cherry on the cake was undoubtedly the 2020 Nobel Prize in Chemistry, which went to Charpentier and Doudna “for the development of a method of genome editing.”
“We are delighted to see that such a remarkable achievement in the area of bioscience has been recognized with this highest honor,” said industry group Europa Bio. “Increased public engagement will facilitate dialogue, sharing of information, and trust-building, which are key for the future advancement of genome editing.”
"* " indicates required fields
"* " indicates required fields
Partner with academic leaders around sustainable materials research
Naive And Primed Pluripotent States "* " indicates required fields